The matter is made up of very small particles called molecules. Molecules are made up of a number of atoms of either the same or different elements which are bonded together. In this post, we shall take a close look at the different types and properties of matter in a few details.
The Greek philosopher called Leucippus and his pupils Democritus (460 – 310 BC) are believed to be the founders of the atomic theory of matter. They speculated that all matter is made up of identical, indivisible particles called atoms separated by empty space.
The existence of molecules suggested by Leucippus and Democritus has been shown by experimental results produced by the English chemist John Dalton in 1808 whose theory proposed that matter was composed of tiny indivisible particles that he then called “ultimate” particles, now known as atoms. His theory further states that every pure substance is made up of a single type of atoms or a combination of different atoms called molecules.
- In 1600’s Boyle tried to explain the observed properties of gases in terms of molecules, their smallest particles (this will be detailed in the gas laws).
- Daniel Bernoulli (1700 – 1782) was the first to explain the law which Boyle discovered. He also related temperature and molecular motion directly, calculated the pressure of a gas from collisions of the molecules with the walls of the containing vessel and inferred that when the temperature increases, the velocity of the molecules increases also.
- The Kinetic Molecular Theory states that “the particles that the matter is made up with are always moving and they often bump into each other”. This random motion is present in varying amount in solids, liquids and gases. The assumptions of kinetic theory of matter can be summarised in the following points:
- All matter is made up of particles called molecules. In normal circumstances it exists in three states.
- All the molecules of a given substance are alike in all respects.
- The molecules are separated from one another by the intermolecular space which is more than the diameter of the molecule itself.
- The molecules attract each other with a force called inter-molecular force, which is strongest in solids and weakest in gases.
- Molecules are in constant motion.
- The temperature of a substance is proportional to the average kinetic energy of all the molecules of the substance.
Table of Contents
States of Matter
Matter exists in the three states of solids, liquids and gas. The physical Properties of Matter in these three states lies in the arrangement and behaviour of their molecules.
These differences can be explained in terms of the Kinetic Theory Model which states that:
- Matter is made up of very small particles called molecules
- These molecules are in constant motion
- The degree of movement which is the measure of kinetic energy depends upon the temperature.
The higher the temperature, the faster the molecules move and therefore the higher the kinetic energy.
Notice that the heat energy of a substance is the total kinetic energy of all the molecules or atoms of that substance.
SOLIDS
In the solid state the molecules are closely packed together and are not free to move about randomly; they vibrate about their fixed position (known as the mean position)

The ability of a solid to maintain its definite shape and fixed volume is the result of the strong intermolecular forces of attraction between the molecules. These are known as cohesive forces.
Solids are classified into two groups: crystalline and non-crystalline (amorphous)
The difference between the two groups is the result of the way in which the molecules are arranged. This molecular arrangement is called the lattice structure of the molecules
Crystalline solids
These are solids whose lattice structure has a definite regular pattern. E.g. sodium chloride (rock salt)

Amorphous solids
The lattice structure of an amorphous solid is an irregular, disordered pattern. E.g.: charcoal and sulphur.
Molecules are arranged differently in different solids and have varying magnitudes of intermolecular forces of attraction. These factors are responsible for some of the mechanical properties of solids (especially metals)
Elasticity: It is the ability of a material to return to its original state or its original shape after it has been stretched or compressed by an external force.
Plasticity: It is the property of a material by which some permanent deformation remains even after the force producing it has been removed.
Ductility: it is the property of a material which governs whether it can be stretched and drawn into wires of small cross-section where there will be considerable deformation without fracture.
Brittleness: This is the property of a material which is fragile and breaks suddenly when a force is applied to it.
Malleability: It is the property which allows a material to be rolled into thin sheets without breaking. E.g. Iron, copper and aluminium can be rolled into thin sheets
Change of solid state:
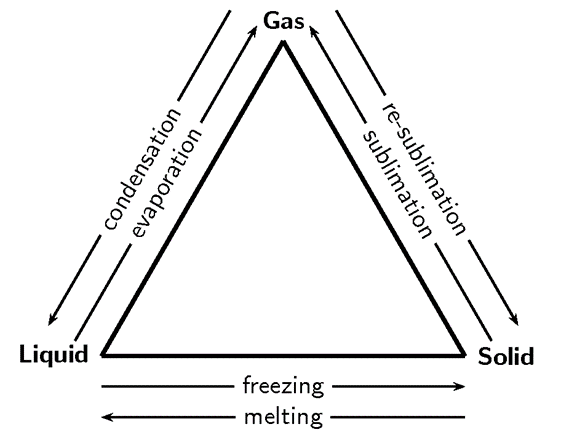
When a solid is heated, the vibrations of molecules and their kinetic energy increase. If the temperature goes to a certain level, the intermolecular forces are broken apart and molecules are released from each other. This results in the material changing its state from a solid to a liquid, the process known as melting or fusion. The opposite phenomenon is called solidification or freezing – change of liquid into solid.
LIQUIDS
Molecules in a liquid are more widely spaced than in a solid, are also vibrating to and fro about their mean position. Their intermolecular force is weak and the molecules movement is free. This enables them to take the shape of the containing vessel.


Under the same temperature conditions, the intermolecular forces of attraction between molecules are different in liquids and this is responsible for the variance in physical properties.
a. Surface tension:
It is the property whereby the molecules on the surface of the liquid do not experience the same intermolecular forces as those within the liquid.
The effect of surface tension
Surface tension tends to reduce the surface area of a liquid. The degree of reduction in the surface area is determined by the surface tension which in turn, depends on the intermolecular forces present. An example of two liquids with different surface tensions is water and mercury:
A droplet of water will tend to spread out, whereas a drop of mercury will be a spherical ball. This is because there is greater surface tension in a mercury droplet than there is in a drop of water.

Another example of the effect of surface tension is the perfectly spherical shape of soap bubbles. Soap solution, detergents and alcohol lower the surface tension of most liquids.
b. Viscosity
This is a property of certain fluids which causes them to offer resistance to the flow of other substances (either solids or liquids) through them.
COHESION AND ADHESION
The particles making substances are held together by intermolecular forces of attraction which are grouped in two major categories according to their action:
- Cohesive forces (cohesion)
It is the intermolecular forces of attraction acting between molecules of the same substance. If these forces are strong, then the surface tension is strong and the surface area of the liquid is reduces.
- When the liquid with very strong cohesive forces are put in a container they form a convexmeniscus.
- When the liquids with weak cohesive forces are put into a container, they form a concave meniscus
2. Adhesive forces (adhesion)
This is the intermolecular forces of attraction acting between molecules of different substances. E.g.: The cohesive forces between the mercury molecules are stronger than the adhesive forces between mercury and glass molecules and subsequently the mercury meniscus in glass container is convex. This is opposite for water and the meniscus are concave. (It is even the reason why water wets glass whilst mercury does not.)

Another example of the effect of cohesion and adhesion is the rise of liquids in very narrow capillary tubes.
Change of liquid state:
When a liquid is heated at high temperature, the particles move faster, intermolecular forces of attraction decrease and intermolecular spaces reduce. This results in the liquid changing the state and turns into gas. This process in known as ‘evaporation’. Evaporation on the surface of a liquid occurs at any temperature but it is very slow. However, when a liquid is boiling, this change takes place everywhere in the liquid and is very fast. The process opposite to evaporation is called condensation – change of gas into liquid.
GASES
In the gaseous state, molecules are even further apart than in water. This implies that they have more freedom to move among themselves. In gaseous state the molecules move haphazardly and at high speed and they are constantly colliding with each other and the walls of the container. These collisions are perfectly elastic, which means that the total kinetic energy of the particles before collision is equal to their total kinetic energy after collision.


Remark: The spaces between the molecules of a gas are relatively large, so that much of the volume occupied by a gas is actually empty space. As a result, the intermolecular forces are almost negligible and the molecules are independent to each other. The increased speeds and the greater spacing between the molecules allow the gas to both fill any volume and also to be compressed (as opposed to expansion).
Change of gaseous state:
When a gas is cooled down, the motion of its particles slows down and the intermolecular spaces reduce. This results in the gas changing into liquid, the process known as condensation or liquefaction. Some substances such as carbon change directly from gas to solid and vice versa. This process, in both cases is called sublimation (e.g.: case of carbon dioxide or dry ice and naphthalene).
The following table summarises properties of gases, liquids, and solids and identifies the microscopic behaviour responsible for each property.
Comparative Characteristics of Gases, Liquids and Solids and their Molecular Explanation. | ||
Gas | Liquid | Solid |
Assumes the shape and volume of its container: particles can move past one another | Assumes the shape of the part of the container which it occupies: particles can move/slide past one another | Retains a fixed volume and shape: rigid – particles locked into place |
Compressible: lots of free space between particles | Not easily compressible: little free space between particles | Not easily compressible: little free space between particles |
Flows easily: particles can move past one another | Flows easily: particles can move/slide past one another | Does not flow easily: rigid – particles cannot move/slide past one another |
Negligible intermolecular forces of attraction between particles. | Weak intermolecular forces of attraction between particles. | Strong intermolecular forces of attraction between particles. |
Table 1.1: Comparison of solid, liquid and gaseous state in terms of their particles’ behaviour
The change of state can be summarised in the following diagram: